In chemical process industries, we come across certain mixtures where separation is impossible or difficult by simple distillation. This type of mixtures we know by the name of azeotropic mixture. Other than this, if relative volatility of the mixture is close to 1.0, in the cases when components boiling points are very close, then also distillation is very difficult. So, in such cases we use azeotropic or extractive distillation techniques. In this article we will focus on azeotropic distillation process for ethanol dehydration.
Table of Contents
What is an Azeotrope?
So, first let us understand what is an azeotrope? In an ideal liquid-vapor systems relative volatility remain constant, in the entire temperature range. When we talk about entire temperature range, it means from component having lowest boiling point to the component having highest boiling point in the mixture.
While in nonideal systems, relative volatility does not remain constant. In other words, relative volatility changes in temperature range. So, when in a nonideal system relative volatility of a mixture is equal to one, mixture at that condition is an azeotropic mixture. We also know this by the name of constant boiling mixture or CBM. This is because when you boil an azeotropic mixture the composition of liquid and vapor remain constant.
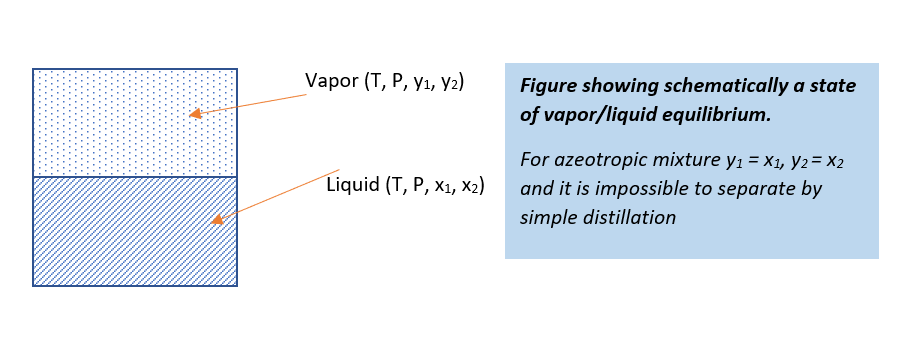
Type of Azeotropes
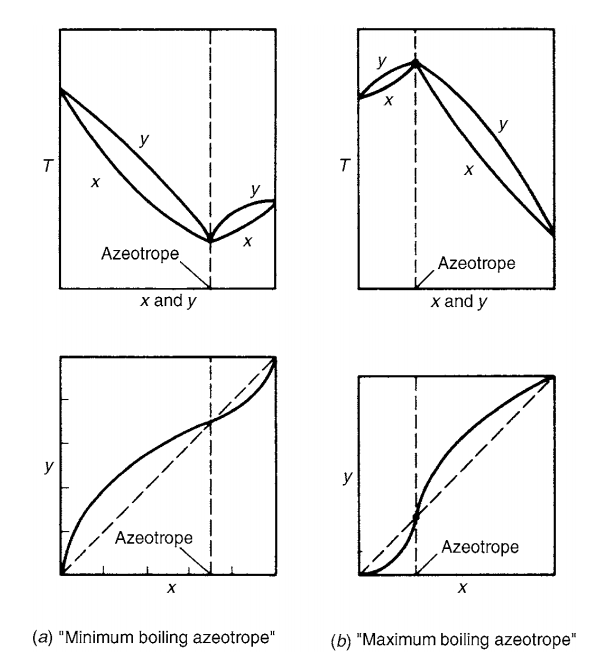
In below figure you can see two types of azeotropic mixture. In first type, when the activity coefficient is greater than unity, this is a positive deviation from Raoult’s law. And, the molecules of components in the system repel each other and exert a higher partial pressure than if their behavior were ideal. This leads to the formation of a “minimum boiling” azeotrope.
While in second type of azeotropes, values of the activity coefficient is less than unity and this we know as negative deviation from Raoult’s law. In such case mixture has lower partial pressure than the ideal mixture behavior. So, this is a formation of a “maximum boiling” azeotrope.
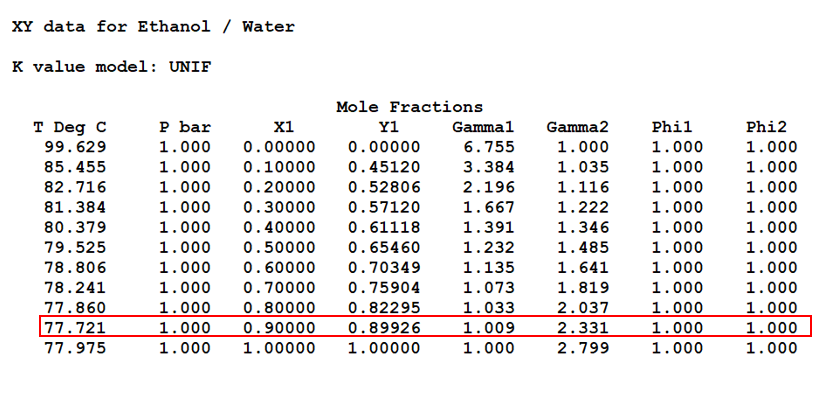
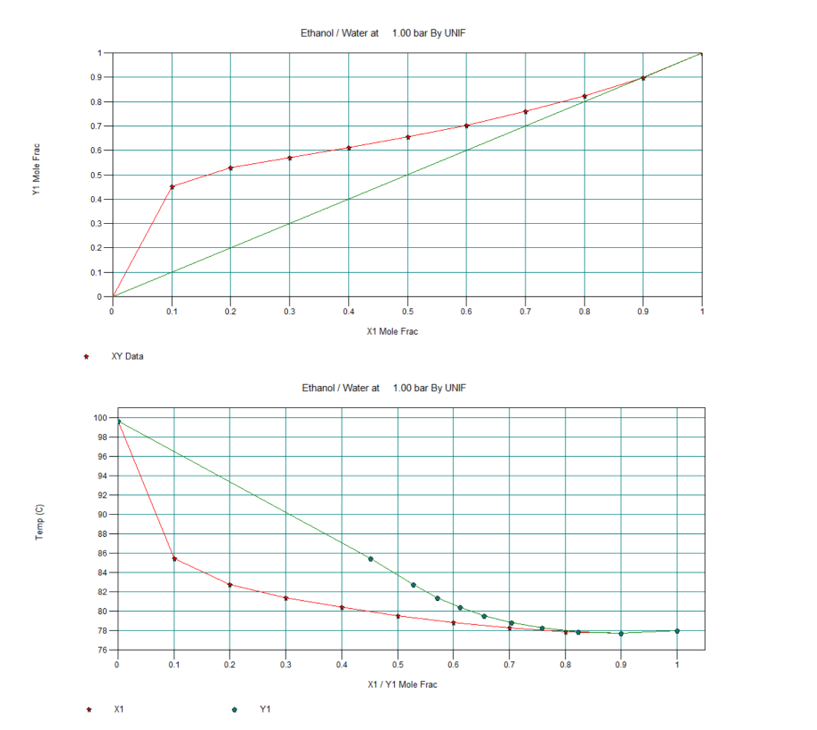
Ethanol T, x, y and x, y Diagrams
In below figure we can see the T, x, y data for Ethanol/Water mixture. Moreover, we can see in red highlighted box this mixture forms an azeotrope at 77.72 0C and 1 bar pressure. At this condition composition in liquid and vapour are same (i.e., x = y = 0.89 mole fraction).
Apart from this in subsequent figures we can see the T, x, y and x, y diagrams also. Therefore, it is impossible to get purity of distilled ethanol more than 0.89 mole fraction by simple distillation. That is the reason from distillery we get this azeotropic mixture, which we call rectified sprit also. The composition of rectified sprit is 89% ethanol and 11% water (by mol), which boils at 77.72 °C. Also please note this is an example of a positive azeotrope.
Also, an azeotrope can be either heterogeneous azeotrope in which two distinguished layer forms which can separated by decantation. While in case of homogenous azeotrope liquid not have separate layer and for separation, we need to add third component. This third component we know as an entrainer and forms a ternary mixture without any azeotropic presence.
How to produce Anhydrous Ethanol?
As we know petrol is a limiting resource of energy, therefore use of anhydrous alcohol can reduce the consumption of petrol. The blended petrol with anhydrous alcohol is known as Power Alcohol, which we can use as a fuel in our vehicles. This power alcohol mixture contains 80% petrol + 20% ethanol and can have various other combinations of concentration.
We can make anhydrous ethanol from azeotropic Ethanol/Water mixture using azeotropic distillation or hydrophilic molecular sieves. In this article we will discuss process for the azeotropic distillation and we will use benzene as an entrainer to break this azeotrope. However, we can use other entrainers also like, cyclohexane, heptane, hexane, toluene, etc.
So, for our case let us consider benzene as an entrainer.
Process Description for Ethanol Dehydration
In for ethanol dehydration process we use three columns, which we can see in below figure. First column is Dehydration Column, second is Benzene Recovery Column and third is Water Removal Column. Therefore, we can divide whole process in three main steps as below.
Ethanol Dehydration Stage
In Dehydration Column, we feed the azeotropic mixture of ethanol and water, which contain 89% ethanol and 11% water on mol basis. In other words, we know this mixture as RS or Rectified Sprit also, which is the final product of a distillery.
So, from dehydration column top ternary azeotrope of benzene with water and alcohol goes to condenser. The boiling point of this azeotrope is 64.9 0C. Subsequently, this ternary mixture after condensation forms two layers. Moreover, for the composition of ternary azeotrope vapor and both the layers, you can refer below table.
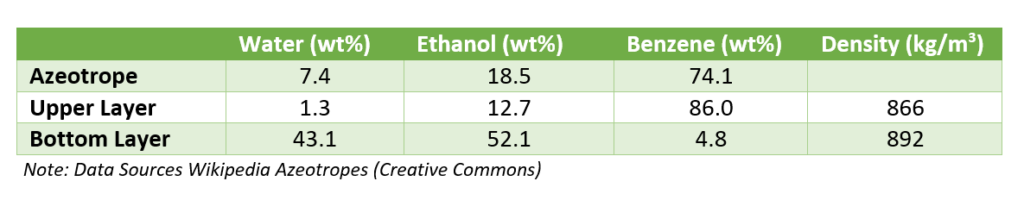
The vapor from benzene recovery column also mix with the vapor of dehydration column. After condensation liquid goes to a decanter. From decanter water rich layer contains small amount of benzene goes to benzene recovery column. While, benzene rich layer is a reflux stream for the anhydrous alcohol column.
The bottom stream of ethanol dehydration column is 99.99% ethanol, which is anhydrous or absolute alcohol.
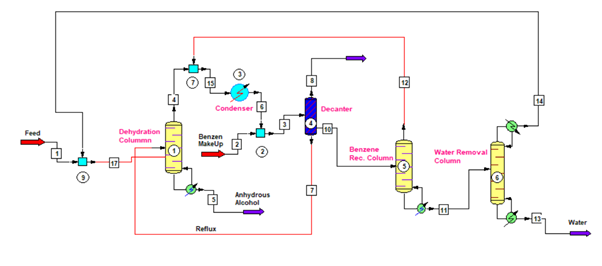
For dehydration column, we can use packed column having structured packing inside for and MOC (material of construction) can be SS304. Moreover, the reboiler, condenser, decanter, all associated piping, valves and fittings should be of SS304 metallurgy.
Recovery of Benzene
For benzene recovery, we use benzene recovery column from the water rich layer. So, from decanter water rich layer stream goes into the benzene recovery column. This column is just like a stripper, which strip out all the benzene from coming feed. The bottom product of this column is free from benzene and this way we recover benzene to reduce the benzene consumption norm. Therefore, reducing anhydrous alcohol manufacturing cost also.
The top stream from this column goes into the anhydrous column condenser. This stream contains more than 50% benzene and balance is ethanol and water. While, the bottom stream of this column is free from benzene and contains ethanol and water only. So, from benzene recovery column bottom, stream goes into the water removal column or ethanol recovery column.
This column is also a packed column and has structured packing. And, the MOC for column and reboiler is SS304. Moreover, piping, valves and other fittings are also made of SS304.
Ethanol Recovery and Water Removal
The purpose of this column is to remove water and recover ethanol from the benzene recovery column bottom stream. This is also a packed column having structured packing inside. Also, the MOC for column, reboiler, condenser and all associated piping, valves and fittings is of SS304.
Bottom stream of this column is as good as pure water and may contain traces of ethanol. This we can recycle inside the plant after suitable treatment. While the top stream is azeotropic mixture of ethanol, which we recycle back into the dehydration column feed tank.
Instrumentation and Control Requirement
So, for the lowest operating cost with safety and longer plant life, an efficient and sustained operation of the plant is very important. For this purpose, we must ensure appropriate instrumentation and controls to maintain the process parameters. Below is the list of required for ethanol dehydration process instrumentation and controls.
Dehydration Column Controls
- Dehydration column feed indication and control valve, to control the feed rate to the column.
- Column level indication and control valve for controlling column level.
- Reflux flow indication and control. This control valve we use to column top temperature also.
- Column reboiler steam flow indication and control valve. We can use this control valve for column bottom temperature control also.
- Decanter interface level indication and control valve. This control is very critical for the efficient operation of the plant. Because any malfunctioning of this control system will disturb the composition of reflux flow stream into the dehydration column. Which will impact the product purity and plant capacity adversely.
- Apart from this we need temperature indication at column top, middle, bottom and pressure indication at column bottom.
Benzene Recovery Column Controls
- Feed flow indication and control valve is required for constant feed flow to the benzene recovery column.
- In benzene recovery column we need reboiler steam feed flow indication and control. This we use to control the heat input to the column and for controlling column bottom temperature also.
- Moreover, we need column level indication and control valve for controlling column level.
- Also, we require temperature indication at column top, middle, bottom and pressure indication at benzene recovery column bottom.
Water Removal Column Controls
- This Feed flow indication and control valve is for constant feed flow to the water removal column. As, constant feed rate is very important for a stable column operation.
- The column reboilers steam feed flow indication and control. This we use to control the heat input to the column and for controlling column bottom temperature also.
- Column level indication and control valve is required for controlling column level control.
- Reflux flow indication and control. This control valve we use to column top temperature also.
- Other then these we need reflux vessel level control valve and indication.
- Moreover, we need temperature indication at column top, middle, bottom and pressure indication at column bottom.
Conclusion
So, we discussed ethanol dehydration process using benzene as an entrainer. However, choice of suitable azeotropic distillation method strongly depends on the separation objective. For example, it may be desirable to recover all constituents of the original feed mixture as a pure component. While in other case only some as pure components and some as azeotropic mixtures suitable for recycle.
Here, to break the ethanol/water azeotrope and in ethanol dehydration process, we exploited liquid-liquid immiscibility of the ternary azeotropic mixture of ethanol, benzene and water.
Moreover, some azeotropes are pressure sensitive and their composition varies with change in pressure. In other words, this we also know as pressure swing distillation process. However, to be a practical pressure swing distillation the change in azeotropic composition must be minimum by 5%, with the change in pressure. Also, there should be moderate pressure change around not more than 10 bar between two pressure points. Example of some applications include the minimum-boiling azeotrope tetrahydrofuran and maximum-boiling azeotropes of hydrogen chloride-water and formic acid-water.
Thanks for reading and looking forward for your valuable feedback.
