As we know, in liquid-liquid extraction process we use solvent, to extract solute from the feed. If we technically see, extraction is the combination of two processes. First is mixing of feed and solvent, while second process is decantation, where phase separation takes place. The solvent and feed are immiscible liquids and forms two distinguished phases during extraction process.
Extraction process works on the solute solubility difference between feed and solvent. Due to solubility difference mass transfer of solute takes place between phases. After extraction, we get solute rich phase, which is known as extract. And, other phase which has generally very low or negligible solute concentration is raffinate.
As you know in chemical process industries, we use various kind of organic solvents like, benzene, ethyl-acetate, heptane, DMF, THF, toluene, methanol, etc. The selection of solvent for extraction depends on the particular process requirements.
Table of Contents
Challenges in Organic Solvent Use
The use of these organic solvents poses challenges like handling and disposal issues. Apart from these, organic solvents create number of environmental concerns, such as atmospheric and land toxicity. Moreover, in many cases, conventional organic solvents are regulated as volatile organic compounds (VOCs). In addition, certain organic solvents are under restriction due to their ozone-layer-depletion potential.
So, to counter above challenges, super critical fluid solvent extraction can be a good alternative. Which you can explore further for your extraction process.
In this article we will discuss in details about the Super Critical Fluid (SFC) and Super Critical Fluid Extraction using CO2.
What is a Super Critical Fluid?
A substance beyond its critical point is known as the Super Critical Fluid (SCF) and in this state compound’s liquid-vapor phase boundary no longer exists. In other words, we can say that beyond critical point for a compound there is no distinction between liquid and vapor phase. To understand this phenomenon, you can refer the below figure.
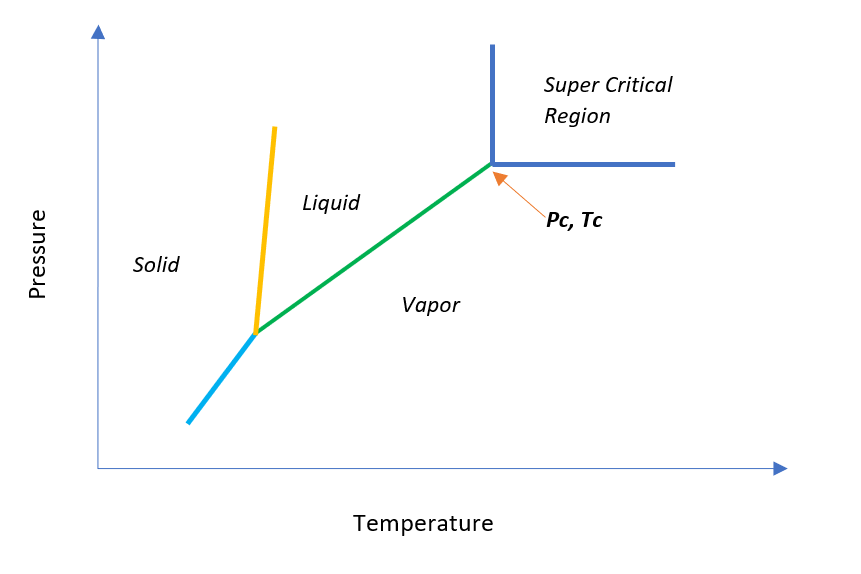
Characteristics of Super Critical Fluid
So, you can see in above T v/s P diagram, above critical point a substance is in super critical region. In this regime fluid properties undergo into remarkable changes and shows properties of liquid and gas both simultaneously. These properties we can list down as below.
- Fluid has liquid like densities
- Surface tension of fluid reduces, which is a liquid property
- Viscosity of SCF is like gas
- Diffusivities are higher than liquids
- SCF has gas like compressibility
Therefore, in nutshell we can say Super Critical Fluid (SCF) is an excellent solvent for extraction process. As they have low surface tension and gas like diffusivities, which is good for higher mass transfer coefficients.
The solvent power of a super critical fluid is approximately proportional to its density. Thus, for SFC solvents we can change the solvent power by varying the temperature and pressure. As SFC solvents are strong function of temperature and pressure therefore, it is very convenient to adjust their properties. On the other side, for conventional organic solvents, you require relatively large pressure changes to change the density.
Various types of Common SCF Solvents
You can find many types of super critical fluid solvents which we use in chemical process industries. These are like CO2, Nitrous Oxide, Water, Ethane, Propylene, Propane, n-heptane, Ethanol, etc.
Below table shows you the critical properties of some SCF solvents.
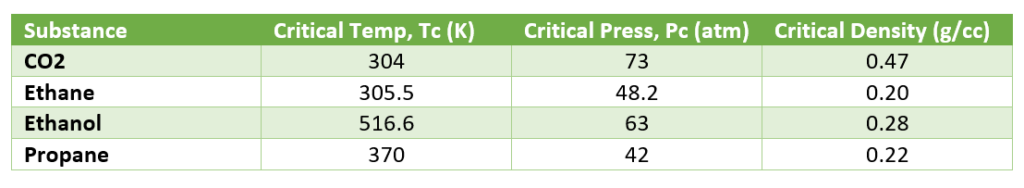
In our chemical processes, we most widely use carbon di-oxide as SCF solvent for the extraction. The CO2 like Pentane and Hexane is very non-polar solvents; therefore, it is best solvent for oils and fats extraction. On other side water, nitrous oxide are polar solvents. And, Ethanol, Methanol and Acetone are in the middle of the polarity scale.
We can use mixture of SCF solvents to enhance the solubility of different solutes.
Why CO2 is the best SCF?
As I mentioned above in our chemical process industry CO2 is best SCF solvent, which we use in extraction process. The reason for this choice is many, which you can see are as follows:
- It has lower critical pressure and temperature, which means comparatively lower operating cost for the extraction process among various other SCF solvents. Moreover, lower pressure and temperature will require lower thickness and pressure rating of equipment. In result, this will reduce overall capital cost of the plant setup.
- Other excellent quality of CO2 is, its relatively non-toxicity and non-flammability. These both properties eliminate the possibilities of any hazardous related to process and people.
- Apart form this CO2 is available at high purity and cost is also low. It is a non-reactive gas.
- Separation of CO2 from extract phase is very easy. We need to just reduce the pressure and this will evaporate out from the mixture. The solubility of CO2 gas is also low or negligible in other chemicals.
- It has polarity like liquid pentane at SCF conditions. Moreover, it is a good solvent for many nonpolar, and a few polar, low-molecular-weight compounds.
Important Parameters for a SCF Extraction Process Design
Now we will discuss about important parameters, which you should consider during the SCF Extraction Process design. We will go through them one by one as below:
- Threshold Pressure of SCF – This is the minimum required pressure at which a solute become soluble in SCF solvent. Generally, when we increase the pressure of a SCF solvent, the solubility of solute increases. But, after a particular pressure, solubility become constant. To understand it, you can refer to below typical diagram.
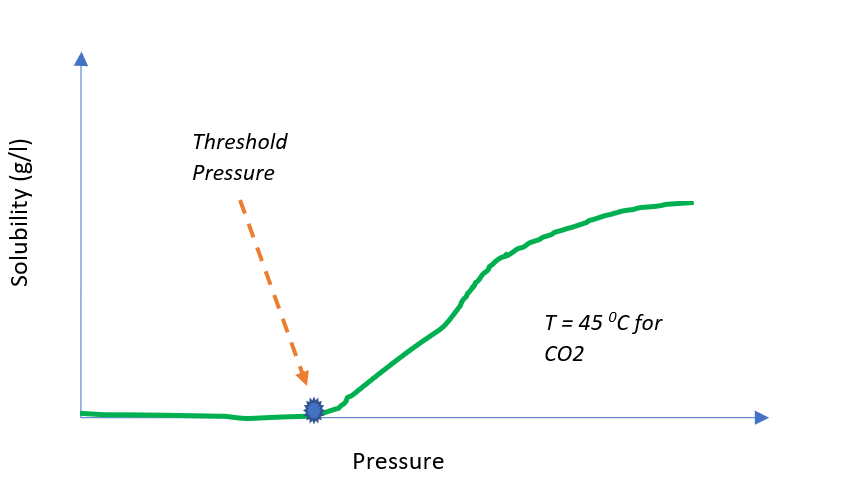
- Second parameter which is important for design point of view is the pressure at which a SCF solvent achieves maximum solubility. Knowing this pressure is important for deciding the pressure rating of equipment. Also, process design calculations will be based on this maximum solubility data.
- Apart from above, knowledge of the physical properties of the solute is very important. Based on solute characteristic various scenarios can be as below:
- There is no interaction between solute and solid phase, the process is dissolution of solute in suitable solvent.
- There can be interaction between solute and solid, in this case extraction is a desorption process. Here, adsorption isotherm of solute on the solid in presence of solvent determines the equilibrium conditions.
- The solid phase swell by the solvent. In that case extraction of entrapped solutes occurs through either dissolution or desorption mechanisms.
- In case is solute is reactive in nature, insoluble solutes react with solvent and products are soluble and we can extract it. The example of this is lignin extraction from cellulose.
Apart from above, other thermodynamic properties which are important for the SCF extraction system design are, temperature, pressure, equation of state, adsorption equilibrium constant, solute solubility, etc.
Process of SCF Solvent Extraction System
This SFC solvent extraction process can be in continuous or batch mode. In most of the cases it is in batch mode or we cab say semi-batch mode. Here, we charge feed in extractor vessel and keep on circulating SFC-CO2 through the extractor till complete solute is extracted from the feed.
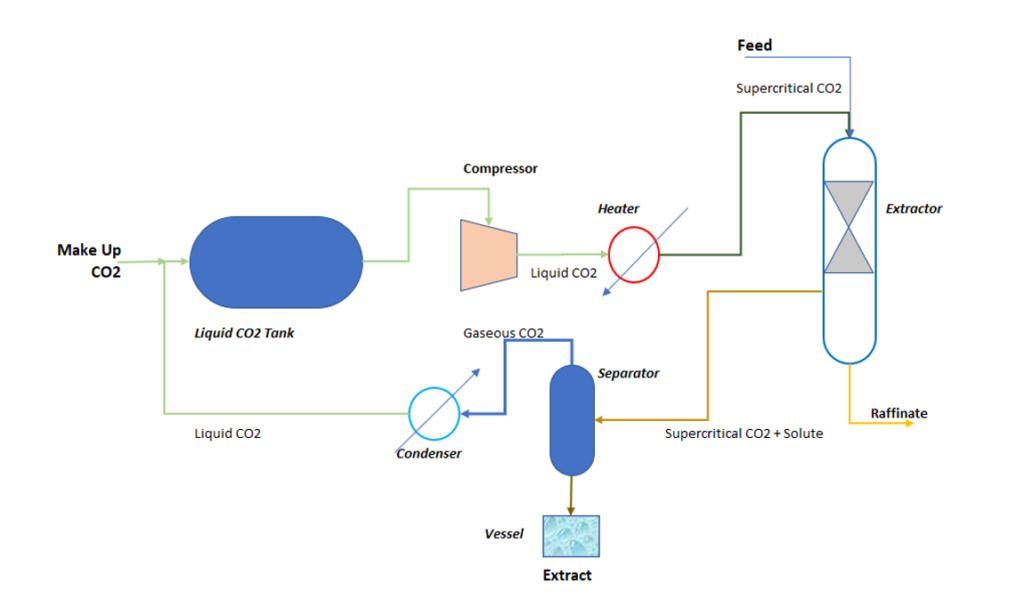
To understand the typical process of SCF Solvent extraction system, you can refer below figure. In this process liquid CO2 enters into a compressor and discharge goes into the heater. Using compressor and heater liquid CO2 is converted into Super Critical Fluid (SCF). Subsequently we mix this SCF CO2 Solvent with feed inside an extractor. This extractor contains packing to provide surface are to enhance the mass transfer rate. In extractor solute transfer takes place from feed to SCF solvent at a particular temperature and pressure conditions.
From extractor, extract phase goes into a separator, while waste stream as raffinate we remove from the extractor. Inside separator vessel, pressure is reduced to a level where, solubility of solute is reduced. Consequently, solute separation takes place and CO2 in gaseous form goes into a condenser. Our product we collect from the bottom of the separator vessel.
The gaseous CO2 condenses into the condenser. And, liquid CO2 again recycle back into the liquid CO2 tank. This way this cycle keeps on continue and any loss of CO2 is fulfilled by a make CO2 gas.
Below are some special applications of SCF solvent extraction process for your reference.
- Extraction of vitamin E from natural resources
- Removal of fat from food
- Removal of pesticides
- Oleoresins extraction for red bell pepper
- Extraction of poisons
- Removal of alcohol from wine
Conclusion
Here we can see SCF solvent extraction has various advantages over conventional organic solvent extraction process. However, major disadvantage in SCF solvent extraction is, it requires high operating pressure. For instance, in case use of SCF-CO2 for Naphthalene extraction operating pressure range is 90 bar to 300 bar.
Therefore, this high pressure needs heavy thickness equipment and high-pressure rating piping, fittings & valves. Moreover, operating cost of the compressor is too high and economic feasibility of the process is under question.
Nevertheless, SCF-CO2 solvent extraction is best suitable for high value and temperature sensitive products. In addition, this provides non-toxic and non-flammable process, which very important in some processes.
Thanks for reading, looking forward for your comments!!!
